Ever wondered whether phosphorus trichloride (PCl₃) is polar or nonpolar? You’re not alone! Many students mix up these chemistry terms because they sound complicated — but they’re actually easy to understand once you know the basics.
In this guide, you’ll learn:
- What polar and nonpolar molecules mean
- The difference between them in simple words
- Why PCl₃ is polar
- How to identify polar vs. nonpolar molecules in your exams or daily study
By the end, you’ll be able to tell the difference between polar and nonpolar molecules instantly — even if chemistry isn’t your favorite subject!
🧪 What Does Each Term Mean?
Let’s start with the basics — what “polar” and “nonpolar” actually mean.
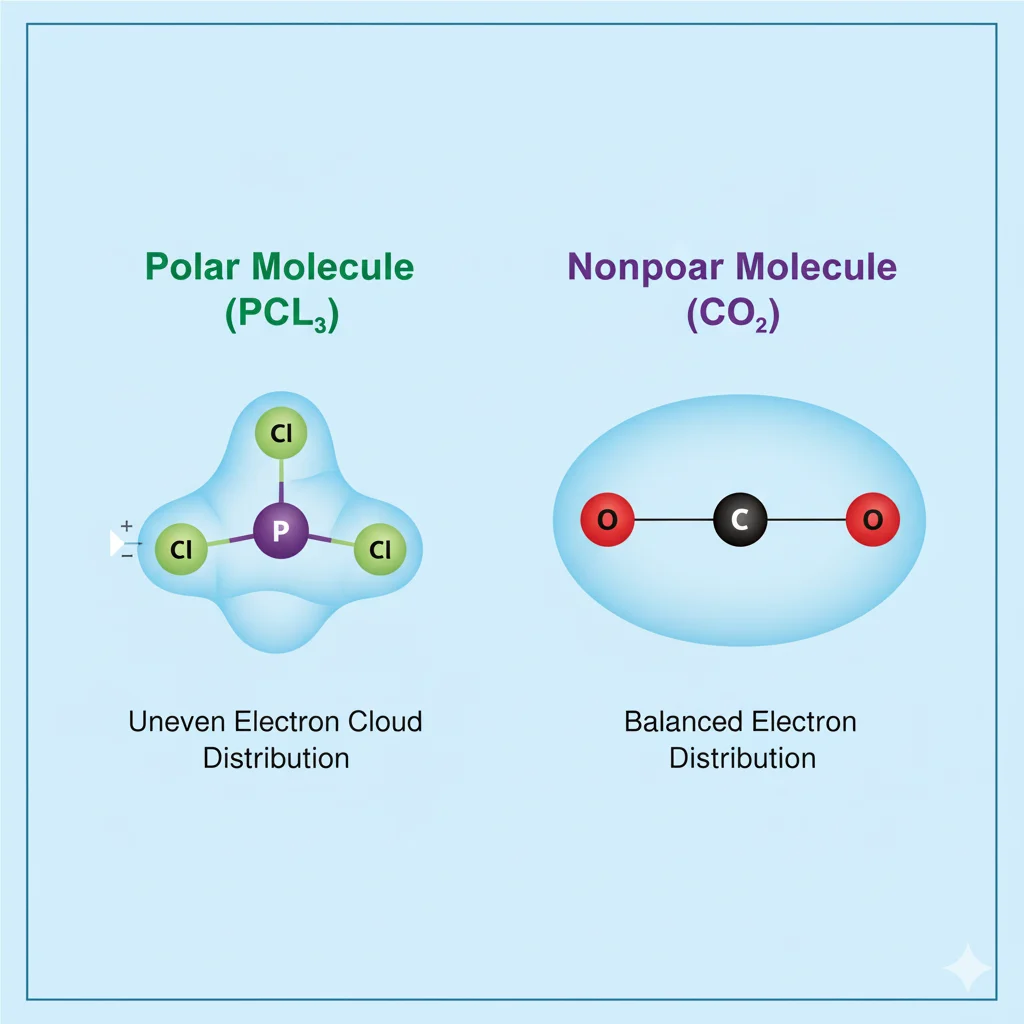
🔹 Polar Molecule
A polar molecule has an uneven distribution of electrons. This means one side of the molecule is slightly negative, and the other is slightly positive — like a tiny magnet!
Examples of polar molecules:
- Water (H₂O)
- Hydrogen fluoride (HF)
- Ammonia (NH₃)
👉 These molecules have atoms that pull electrons with different strengths, creating a “dipole” or a partial charge difference.
🔸 Nonpolar Molecule
A nonpolar molecule shares electrons evenly between its atoms. There’s no positive or negative side, so it doesn’t act like a magnet.
Examples of nonpolar molecules:
- Oxygen (O₂)
- Carbon dioxide (CO₂)
- Methane (CH₄)
👉 In these molecules, the pull between atoms is balanced — so the molecule stays neutral all over.
⚖️ The Key Difference Between Polar and Nonpolar
| Feature | Polar Molecule | Nonpolar Molecule |
|---|---|---|
| Electron Sharing | Uneven | Even |
| Charge Distribution | Has positive and negative sides | No charge difference |
| Shape | Often bent or asymmetrical | Usually symmetrical |
| Example | Water (H₂O), PCl₃ | CO₂, CH₄ |
| Attraction | Strong between molecules | Weak between molecules |
💡 Quick Tip:
If a molecule’s shape isn’t symmetrical and it has atoms with different electronegativities (pulling power), it’s polar.
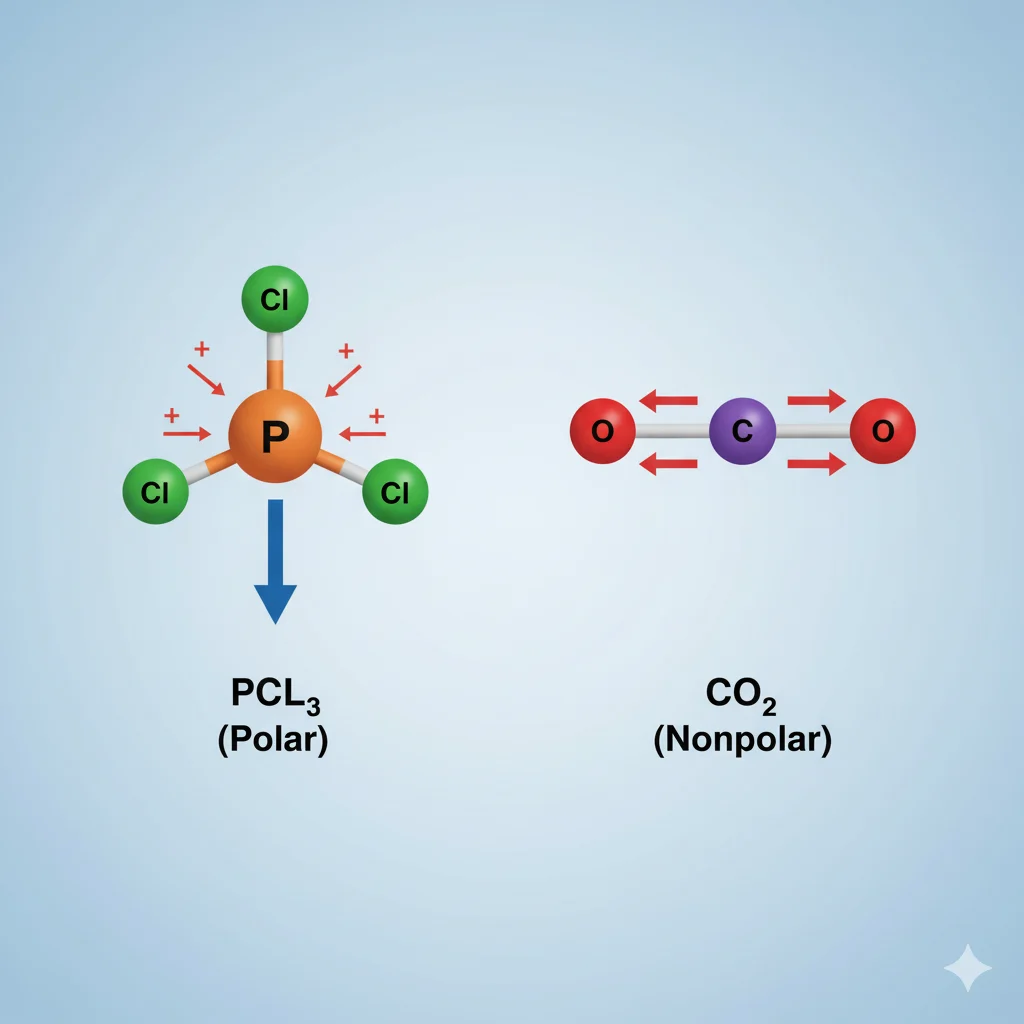
💧 Is PCl₃ Polar or Nonpolar?
The molecule phosphorus trichloride (PCl₃) is polar.
Here’s why:
- Shape: PCl₃ has a trigonal pyramidal shape (like a pyramid with phosphorus in the center and three chlorine atoms at the corners).
- Lone Pair: Phosphorus has one lone pair of electrons on top, which makes the molecule asymmetrical.
- Electronegativity Difference: Chlorine pulls electrons more strongly than phosphorus, creating an uneven charge distribution.
➡️ So, electrons are pulled more toward the chlorine atoms, giving PCl₃ a slightly negative side near the chlorines and a slightly positive side near the phosphorus.
✅ Conclusion: PCl₃ is a polar molecule because it has an uneven distribution of charge due to its shape and electron arrangement.
⚠️ Common Mistakes and How to Avoid Them
Here are some mistakes students make when deciding if a molecule is polar or nonpolar:
❌ Mistake 1:
Assuming that all molecules with the same atoms are nonpolar.
✅ Correction:
Even if all atoms are the same, the shape can make a molecule polar (like H₂O).
❌ Mistake 2:
Ignoring the lone pairs on the central atom.
✅ Correction:
Lone pairs can bend the molecule’s shape, creating polarity (like in PCl₃ and NH₃).
❌ Mistake 3:
Focusing only on electronegativity, not on shape.
✅ Correction:
Both shape and electronegativity difference decide polarity.
🔹 When to Classify a Molecule as Polar
You should classify a molecule as polar when:
- It has uneven electron sharing.
- Its shape is asymmetrical.
- The atoms have different pulling strengths.
Examples:
- Water (H₂O) → Bent shape → Polar
- Ammonia (NH₃) → Pyramidal → Polar
- Phosphorus trichloride (PCl₃) → Pyramidal → Polar
🔸 When to Classify a Molecule as Nonpolar
A molecule is nonpolar when:
- It shares electrons equally.
- Its shape is symmetrical.
- The atoms have equal pulling strengths.
Examples:
- Carbon dioxide (CO₂) → Linear → Nonpolar
- Oxygen (O₂) → Two same atoms → Nonpolar
- Methane (CH₄) → Tetrahedral and symmetrical → Nonpolar
💡 Memory Hack:
“If it’s even and symmetrical, it’s nonpolar.
If it’s uneven or bent, it’s polar.”
🔁 Quick Recap: Polar vs. Nonpolar
- Polar molecules have uneven charge distribution and an asymmetrical shape.
- Nonpolar molecules have even charge distribution and symmetrical shapes.
- PCl₃ is polar because of its shape and electronegativity difference.
- Always check both shape and electronegativity before deciding.
🧠 Advanced Tips
- Origin: The idea of polarity comes from how molecules share electrons — a key concept in chemical bonding.
- In Exams: When asked “Is PCl₃ polar or nonpolar?”, always mention:
- Shape: Trigonal pyramidal
- Lone pair: Yes (1 on phosphorus)
- Result: Uneven charge → Polar molecule
- In Real Life: Polarity affects how substances dissolve. For example, polar molecules like PCl₃ dissolve in water (a polar solvent), while nonpolar molecules dissolve in oil.
✏️ Mini Quiz: Test Yourself
Fill in the blanks:
- PCl₃ has a __________ shape.
- A molecule is polar if it has __________ electron sharing.
- Nonpolar molecules have __________ shapes.
- The main reason PCl₃ is polar is because of its __________ pair on phosphorus.
- CO₂ is __________ because it has a symmetrical shape.
(Answers: 1. Trigonal pyramidal, 2. Uneven, 3. Symmetrical, 4. Lone, 5. Nonpolar)
❓ FAQs
1. Is PCl₃ polar or nonpolar?
PCl₃ is polar because of its pyramidal shape and uneven electron distribution.
2. What makes a molecule polar?
A molecule is polar if it has a difference in electronegativity and an asymmetrical shape, creating positive and negative ends.
3. Can a molecule with the same atoms be polar?
Yes, if its shape causes uneven electron distribution — like in H₂O.
4. Is PCl₃ soluble in water?
Yes, polar molecules like PCl₃ dissolve well in polar solvents such as water.
5. How can I quickly tell if a molecule is polar or nonpolar?
Check two things — the shape (symmetrical or not) and electronegativity difference. If both are uneven, it’s polar.
🧾 Conclusion
So, is PCl₃ polar or nonpolar?
The answer: PCl₃ is a polar molecule.
Because of its asymmetrical (pyramidal) shape, lone pair on phosphorus, and unequal electron pull, it creates a dipole moment — one side slightly negative, one slightly positive.
Now that you understand the difference between polar and nonpolar molecules, you can easily spot them in your chemistry lessons — and never get confused again!
Keep practicing and exploring — science becomes simple when you learn step by step! 🌟

Arwen Blythe is a passionate language and culture enthusiast, crafting clear, engaging guides on words, phrases, and modern English for Definevs.com readers.