Salt water is homogeneous because the salt is evenly dissolved and you cannot see or separate the parts by simple observation. However, it can become heterogeneous if the salt does not fully dissolve or if sand, dirt, or other particles mix with it.
People often ask “Is salt water homogeneous or heterogeneous?” because the answer feels tricky at first. In daily life, we see salt water at beaches, in cooking, and even in tears. But when we talk about types of mixtures in science, we need to understand whether salt water behaves like one uniform substance or a mix we can notice by eye.
In this simple, friendly guide, you will learn:
- the meaning of homogeneous and heterogeneous in easy words
- why salt water is usually homogeneous
- when salt water becomes heterogeneous
- examples, comparison tables, and mini science tips
- a quick recap and a short quiz to check your understanding
By the end, even a 4th-grade student will clearly understand the difference between salt water as a homogeneous or heterogeneous mixture and will be able to explain it with confidence.
What Do “Homogeneous” and “Heterogeneous” Mean?
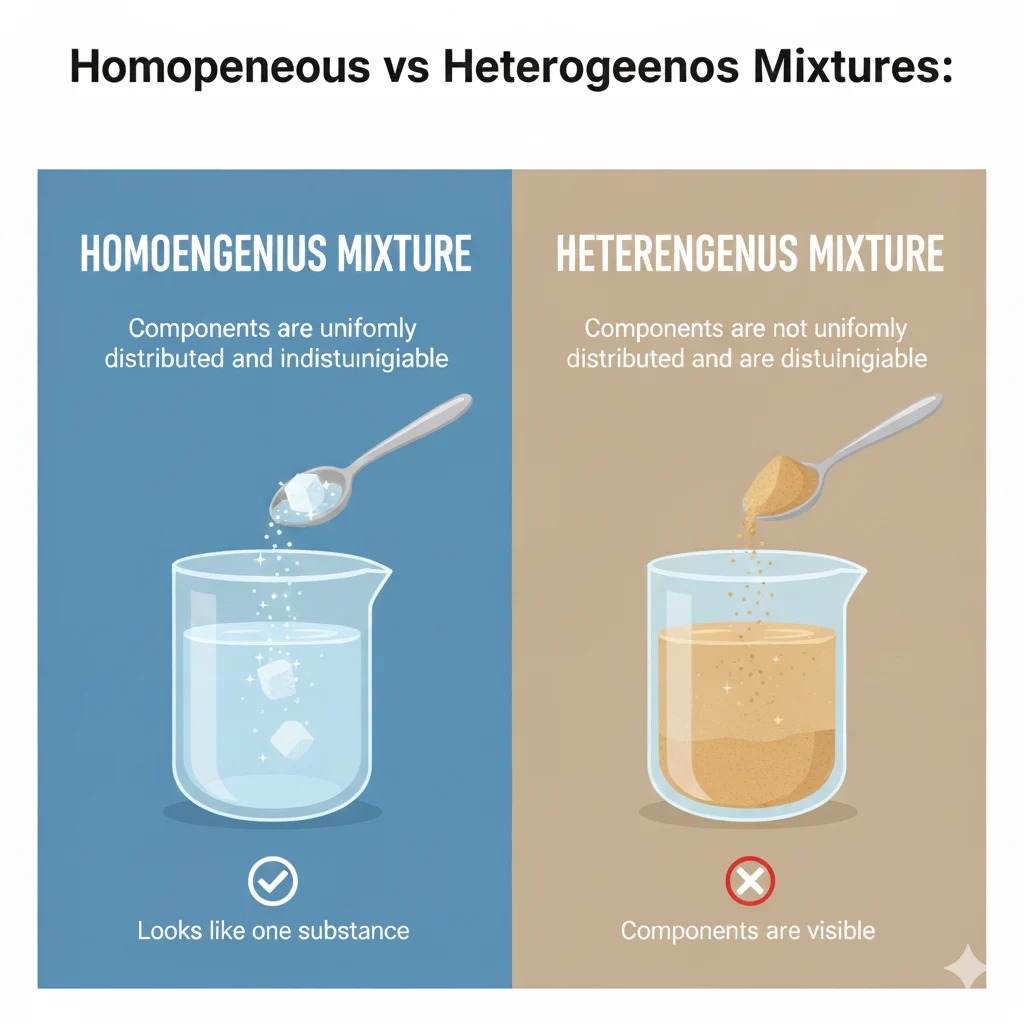
Before we decide if salt water is homogeneous or heterogeneous, we must understand both terms.
These words come from science but can be explained in a very simple way.
Homogeneous
A homogeneous mixture looks the same everywhere.
You cannot see or pick out different parts with your eyes.
| Key Idea | Looks the same everywhere |
|---|---|
| Can you see parts? | No |
| Can you separate by hand? | No |
| Example | Sugar dissolved in tea |
Examples of homogeneous mixtures:
- Sugar water
- Lemon juice without pulp
- Air (all gases mixed evenly)
Heterogeneous
A heterogeneous mixture looks different in different places.
You can see or separate the parts easily.
| Key Idea | Looks different in places |
|---|---|
| Can you see parts? | Yes |
| Can you separate by hand? | Yes |
| Example | Sand in water |
Examples of heterogeneous mixtures:
- Oil and water
- Sand in water
- Pizza toppings
The Key Difference Between Homogeneous and Heterogeneous (Easy Table)
| Feature | Homogeneous Mixture | Heterogeneous Mixture |
|---|---|---|
| Looks like | One thing | Many things |
| Can you see parts? | No | Yes |
| Can you separate by hand? | No | Often yes |
| Mixes evenly? | Yes | No |
| Salt water | Most often Yes | Sometimes, depending on added materials |
Quick Tip:
If it looks like one thing, think homogeneous.
If it looks like many things, think heterogeneous.
So… Is Salt Water Homogeneous or Heterogeneous?
➡️ Salt water is homogeneous most of the time
because when salt dissolves completely in water, it spreads evenly.
You cannot see the salt grains, and the taste is the same everywhere.
But…
➡️ Salt water becomes heterogeneous
if salt does not fully dissolve or extra materials like sand, dirt, algae, or mud mix in.
Examples:
- Homogeneous salt water: Water used for saline medicine
- Heterogeneous salt water: Seawater with floating shells and sand
Why People Get Confused (Common Mistakes & Fixes)
People often think salt water is always heterogeneous because:
- beach water contains sand, shells, and dirt
- they can taste salt and think tasting means separation
- they see salt crystals before they dissolve
Common Incorrect Idea
❌ Salt water is heterogeneous because I can taste the salt.
Correct Idea
✔️ Even though you taste salt, you do not see it. The salt is evenly dissolved, making the mixture homogeneous.
When to Call Salt Water a Homogeneous Mixture
Use the word homogeneous when:
- the salt is fully dissolved
- the water looks clear (even if salty)
- you cannot see salt grains
- the mixture tastes the same everywhere
Real-life examples:
- Cooking: Salt dissolved in soup
- Sports: Saline water to relieve dehydration
- Hospital: Saline drips for patients
- Experiments: Clean salt solution in science labs
- Everyday: Tears are naturally salty water
When to Call Salt Water a Heterogeneous Mixture
Use the word heterogeneous when:
- the salt does not fully dissolve
- there are extra materials like dirt or sand
- the water looks cloudy or chunky
Real-life examples:
- Beach water with sand and small stones
- Water left in a bucket with salt at the bottom
- Salt water mixed with seaweed or algae
- Dirty aquarium salt water
- Salt water containing rust particles
Memory Trick
“Clear and salt = homogeneous.
Cloudy and salty = heterogeneous.”
Quick Recap: Homogeneous vs Heterogeneous
- Salt water is usually homogeneous ✔️
- It only becomes heterogeneous if extra particles are present
- Homogeneous = even mixture (like clear salty water)
- Heterogeneous = uneven mixture (like sea water with sand)
- Look first: If you see parts, it’s heterogeneous
If it looks like one thing, it’s homogeneous
Advanced Tips
- Scientists classify mixtures to predict behavior, like boiling point or freezing point
- Salinity in oceans is mostly homogeneous in small areas, but changes with depth
- In research and chemistry, salt water is called a solution, meaning one substance dissolves into another
- Misunderstanding the terms can cause mistakes in science answers or project reports
- Writing “salt water is always heterogeneous” in exams is incorrect unless you mention impurities
Mini Quiz (Test Yourself!)
Fill in the blanks:
- Salt water is usually a __________ mixture because the salt dissolves evenly.
- If salt remains at the bottom, the water becomes __________.
- A mixture that looks like one thing is called __________.
- Sea water with sand and shells is __________.
- Lemon juice without pulp is a __________ mixture.
- Oil and water form a __________ mixture.
- When extra particles enter salt water, it becomes __________.
Answers
- homogeneous
- heterogeneous
- homogeneous
- heterogeneous
- homogeneous
- heterogeneous
- heterogeneous
FAQs
1. Is salt water homogeneous or heterogeneous?
Mostly homogeneous, unless extra particles make it uneven.
2. Why can’t I see the salt in salt water?
Salt dissolves completely and spreads evenly, making it invisible.
3. Can salt water be separated?
Yes, by evaporation — the water evaporates and salt stays behind.
4. Is sea water homogeneous or heterogeneous?
Mostly heterogeneous because of sand, shells, and sea life.
5. What type of mixture is clean salt water?
A homogeneous solution.
Conclusion
Now you clearly understand whether salt water is homogeneous or heterogeneous. Most of the time, salt water is homogeneous because the salt dissolves completely, creating a smooth, even mixture. But once extra particles join in, the same water becomes heterogeneous.
This simple rule helps you answer science questions correctly and understand mixtures in real life—from cooking to swimming.
Whenever you see salt water, try asking yourself:
“Do I see different parts or not?”
Your answer will tell you the correct mixture type.
Keep exploring, stay curious, and soon science will feel simple and fun every day!

Kael Donovan is a language enthusiast and writer at Definevs.com, simplifying complex words and grammar rules into fun, easy-to-understand guides for readers.









