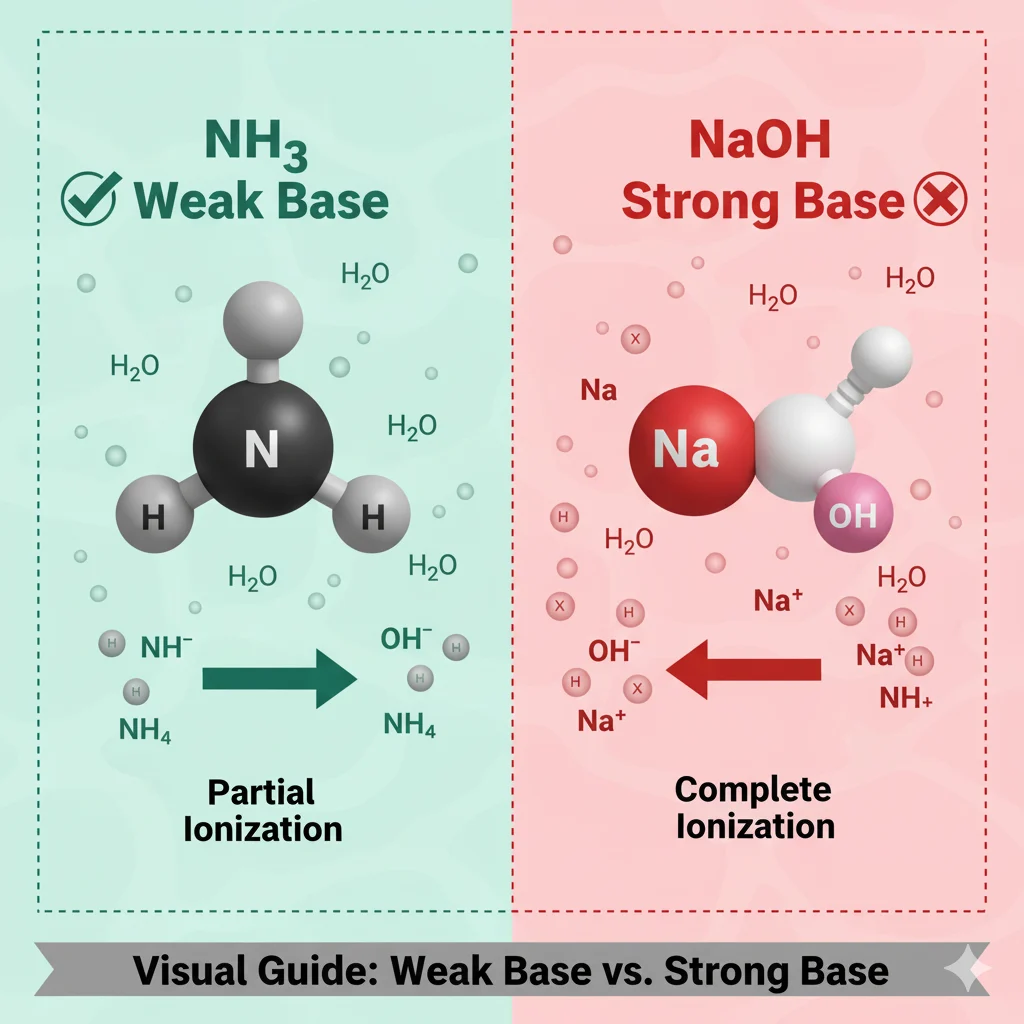
NH₃ (ammonia) is a weak base, not a strong base.
It does accept protons, but it doesn’t ionize completely in water — which is why chemists call it a weak base.
Have you ever sat in science class and wondered, “Is NH₃ strong or weak?” You’re not alone! This is one of the most confusing chemistry questions for students because NH₃ behaves like a base — but not in the dramatic, powerful way that strong bases do.
The good news? This guide breaks everything down in the simplest way possible. If you can understand everyday things like sugar dissolving or salt melting, you can understand whether NH₃ is strong or weak, what it means, and how to use the idea correctly in homework, exams, and daily understanding.
In this guide, you’ll learn:
- What strong and weak bases mean
- What NH₃ does in water
- The difference between strong and weak bases
- Easy examples and memory tricks
- Common mistakes students make
By the end, even a 4th-grade student could explain it confidently!
🔍 What Does “Strong” Mean vs. What Does “Weak” Mean? (Chemistry Made Simple)
To understand whether NH₃ is strong or weak, we must know what these two words mean in chemistry.
1. What Does “Strong” Mean?
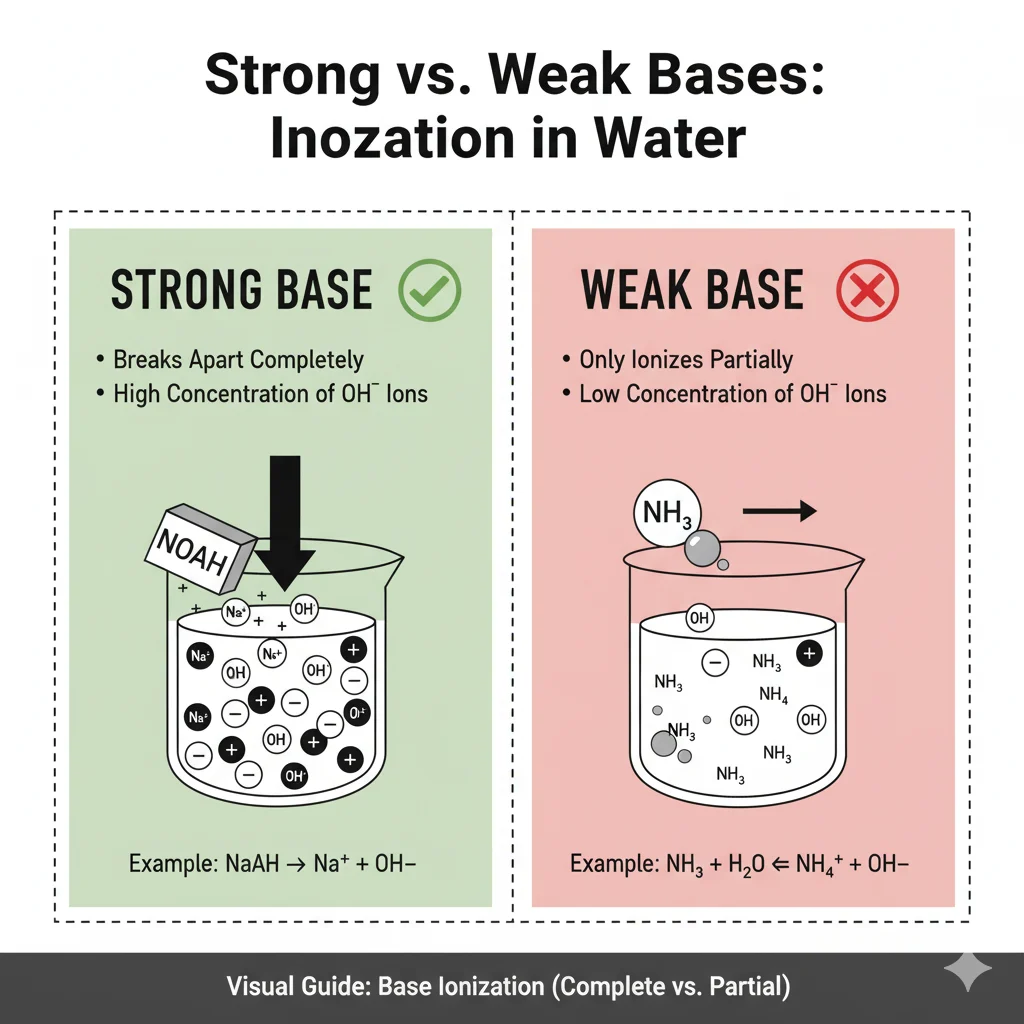
A strong base is one that breaks apart completely in water.
This means all its molecules split and make ions.
Simple example:
Imagine a cookie that falls into crumbs the moment it touches milk — that’s a strong base.
Examples:
- NaOH (sodium hydroxide)
- KOH (potassium hydroxide)
2. What Does “Weak” Mean?
A weak base only breaks apart a little bit in water.
Some molecules split, but many stay whole.
Simple example:
A cookie that stays mostly whole even when soaked — only a few crumbs fall off.
Three Easy Examples to Remember (Strong vs. Weak)
Strong Base Example Sentences:
- “NaOH breaks apart completely — it’s very strong.”
- “Strong bases make lots of OH⁻ ions.”
- “Strong bases react quickly and strongly in water.”
Weak Base Example Sentences:
- “NH₃ breaks apart only a little — that makes it weak.”
- “Weak bases make fewer ions.”
- “Weak bases react slowly and gently.”
⚖️ Is NH₃ Strong or Weak? The Key Difference Explained
Answer:
NH₃ is a weak base, not a strong base.
Why?
Because when NH₃ dissolves in water, only some of its molecules grab hydrogen ions (H⁺).
Most NH₃ molecules stay the same.
Here’s what happens:
NH₃ + H₂O → NH₄⁺ + OH⁻
But this reaction doesn’t happen a lot — only a small percentage reacts.
That’s why NH₃ is called weak.
Comparison Table — Strong vs. Weak Base (Using NH₃)
| Feature | Strong Base | Weak Base (Like NH₃) |
|---|---|---|
| Breaks apart in water | Completely | Partially |
| Ion production | High | Low |
| Strength | Very high | Lower |
| Example | NaOH | NH₃ |
| Reaction speed | Fast & strong | Mild & slow |
⭐ Quick Tip to Remember
If it doesn’t break apart fully in water → it’s weak. NH₃ does NOT break apart fully → NH₃ is weak.
❌ Common Mistakes Students Make (and How to Fix Them)
Mistake 1: Thinking “NH₃ smells strong, so it must be a strong base.”
Fix: Smell has nothing to do with chemical strength. Strength refers to ionization, not smell.
Mistake 2: Believing NH₃ is strong because it reacts with acids.
Fix: Many weak bases still react with acids — that doesn’t make them strong.
Mistake 3: Thinking weak bases are useless or “bad.”
Fix: Weak and strong bases are just different, not better or worse.
🧪 When to Call NH₃ a Weak Base (Clear Situations & Examples)
Use the term “weak base” when:
1. Talking about water reactions
Example:
“NH₃ forms only a small amount of OH⁻ in water, so it’s a weak base.”
2. Talking about ionization
Example:
“NH₃ does not ionize completely.”
3. Comparing with strong bases
Example:
“KOH is stronger than NH₃.”
4. Doing chemistry homework or exams
Example:
“NH₃ is a weak base commonly found in fertilizers.”
5. Explaining how bases behave in real life
Example:
“NH₃ is weak but still useful in cleaning products.”
🧪 When Not to Call NH₃ Strong (Easy Practical Guide)
NH₃ should NOT be called a strong base when:
- Talking about complete ionization
- Comparing with NaOH, KOH, or Ca(OH)₂
- Explaining pH levels
- Discussing lab reactions where strong bases act faster
- Doing multiple-choice questions (MCQs)
Memory Hack:
Strong = Complete
Weak = Incomplete
NH₃ = incomplete → weak.
🔁 Quick Recap: Is NH₃ Strong or Weak?
- NH₃ is a weak base.
- It does not ionize completely in water.
- Strong bases break apart fully → NH₃ does not.
- NH₃ reacts, but not strongly.
- Weak ≠ useless. Weak just means “not fully ionized.”
📘 Advanced Tips (Simple but Helpful)
Where the term comes from:
“Base strength” describes how many ions a compound makes in water.
In exams or essays:
Always write:
“NH₃ is a weak base because it partially ionizes in water.”
In real life:
Weak bases like ammonia are used in:
- Cleaning sprays
- Fertilizers
- Refrigeration
- Household products
Even though it’s weak, NH₃ is very useful.
📄 FAQs
1. Why is NH₃ a weak base?
Because it only reacts a little bit in water and produces fewer OH⁻ ions compared to strong bases.
2. Is NH₃ strong or weak in water?
Weak. It does not fully ionize in water, so chemists call it a weak base.
3. Does NH₃ raise pH?
Yes, but not as much as strong bases. It increases pH a little.
4. Is NH₃ stronger than NaOH?
No. NaOH is a strong base. NH₃ is weak because it ionizes partially.
5. Can NH₃ act like a strong base sometimes?
In some reactions it behaves strongly, but in water it is always classified as a weak base.
📝 Conclusion
Now you know exactly why NH₃ is a weak base. Even though it reacts with acids and is widely used in everyday life, it does not fully break apart in water — which is the key feature of strong bases. By understanding how strong and weak bases work, chemistry becomes much simpler and less confusing.
Keep practicing, keep checking examples, and soon you’ll understand every base, acid, ion, and reaction with complete confidence. Great job learning something new today — you’re getting better at science every day!

Arwen Blythe is a passionate language and culture enthusiast, crafting clear, engaging guides on words, phrases, and modern English for Definevs.com readers.