Ice melting is endothermic — it absorbs heat from its surroundings to change from solid to liquid.
It is not exothermic, because no heat is released during melting.
Many students wonder: is ice melting endothermic or exothermic? These two words sound scientific and confusing, so people easily mix them up. Both terms explain what happens to heat and energy during different processes. But when someone sees ice melting in a drink, they often ask whether heat is being taken in or given out.
In this simple guide, you will learn the meaning, difference, and correct usage of the terms endothermic and exothermic, using real-life examples you already understand.
We’ll break everything down so even a 4th-grade student can explain the answer.
By the end of this article, you will never confuse these two words again. You’ll know why ice melting is endothermic, how to use these terms correctly, and how to remember them with easy tricks. Let’s make science simple and fun!
What Do Endothermic and Exothermic Mean?
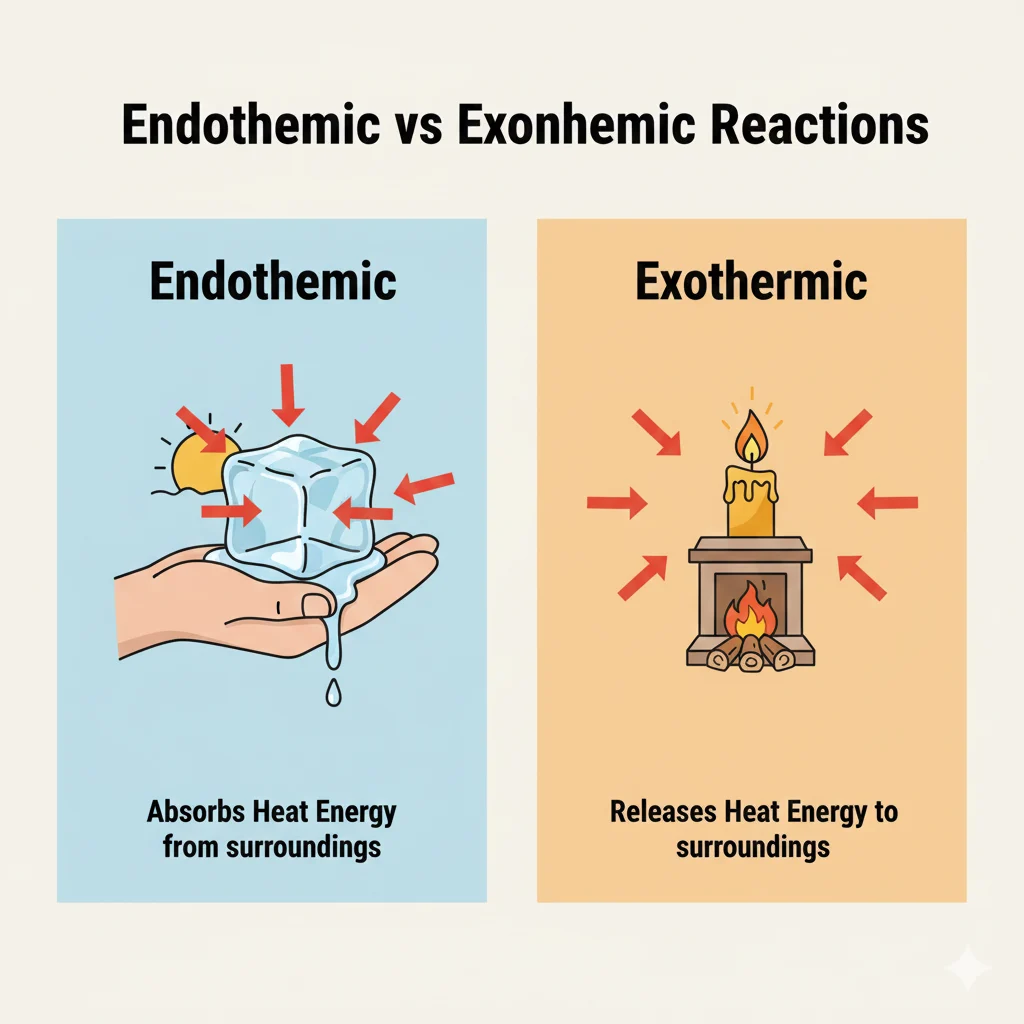
Before we explore is ice melting endothermic or exothermic, we must clearly understand both words.
Endothermic — Meaning & Simple Definition
- Part of speech: adjective
- Meaning: A process that absorbs heat from the surroundings.
Easy examples:
- Ice melting in your hand — it feels cold because it takes heat from your skin.
- Butter melting on hot toast — heat is absorbed to turn solid to liquid.
- Water evaporating from clothes — heat is absorbed to turn liquid into gas.
Exothermic — Meaning & Simple Definition
- Part of speech: adjective
- Meaning: A process that releases heat into the surroundings.
Easy examples:
- A burning candle — gives out heat and light.
- Fireworks exploding — energy is released quickly.
- Hand warmers — they heat up when chemicals react inside.
The Key Difference Between Endothermic and Exothermic
To answer our main question—is ice melting endothermic or exothermic?—let’s compare both side-by-side.
| Feature | Endothermic | Exothermic |
|---|---|---|
| Heat movement | Absorbs heat | Releases heat |
| Temperature of surroundings | Goes down | Goes up |
| Example in daily life | Ice melting | Fire burning |
| Phase change example | Solid → Liquid (melting) | Liquid → Solid (freezing) |
| How your hand feels | Cold (loses heat) | Warm (gains heat) |
| Visual memory trick | “Endo = Enter heat” | “Exo = Exit heat” |
Quick Tip to Remember
- Endothermic: heat goes IN → like IN-dothermic
- Exothermic: heat goes OUT → like EX-it-thermic
Common Mistakes and How to Avoid Them
| Incorrect Sentence | Correct Sentence | Why? |
|---|---|---|
| Ice melting gives off heat. | Ice melting absorbs heat. | Melting needs heat to break particles apart. |
| Freezing water is endothermic. | Freezing water is exothermic. | Heat is released when liquid turns solid. |
| Burning wood takes in heat. | Burning wood releases heat. | Fire gives out heat and light. |
Why mistakes happen:
Many people think melting gives heat because warmth is involved. But actually, ice absorbs heat to melt, which makes it endothermic.
When to Use “Endothermic”
Use endothermic when heat is absorbed to make a change happen.
Examples:
- Ice melting on the road absorbs sunlight.
- Chocolate melting in your hand takes heat from your skin.
- Water boiling needs heat from the stove.
- Photosynthesis in plants absorbs sunlight.
- Evaporation after rain absorbs heat from the ground.
Memory Hack
“Endo = Enter” → heat enters the object.
When to Use “Exothermic”
Use exothermic when heat is released during the process.
Examples:
- Campfire warming your hands releases heat.
- Freezing water into ice releases heat to the air.
- Rusting iron slowly gives off heat.
- Firecrackers popping release energy suddenly.
- Baking soda + vinegar reaction can release heat.
Memory Hack
“Exo = Exit” → heat exits the object.
Quick Recap: Endothermic vs Exothermic
- Endothermic = heat in
- Exothermic = heat out
- Ice melting = Endothermic
- Freezing water = Exothermic
- Burning wood = Exothermic
- Evaporation = Endothermic
If heat goes into something, it’s endothermic.
If heat comes out, it’s exothermic.
Is Salt Water Homogeneous Or Heterogeneous? shocking real answer 💥🌍
Advanced Tips
Origin of the Words
- “Endo” comes from Greek meaning inside.
- “Exo” comes from Greek meaning outside.
Academic Usage
In essays, exams, and science tests, always mention:
- Heat direction (in or out)
- Example process
- Temperature effect
Texting & Online Writing
People sometimes write:
- “Ice melting releases heat” — ❌ incorrect
- It can make the science meaning unclear.
Mini Quiz: Test Yourself!
Fill in the blanks with endothermic or exothermic:
- Burning coal is ____________.
- Ice melting in soda is ____________.
- Freezing water into ice cubes is ____________.
- Water boiling on a stove is ____________.
- Fireworks exploding are ____________.
- Evaporation of sweat is ____________.
- Hand warmers are ____________.
Answers
- Exothermic
- Endothermic
- Exothermic
- Endothermic
- Exothermic
- Endothermic
- Exothermic
FAQs
1. Is ice melting endothermic or exothermic?
Ice melting is endothermic because it absorbs heat.
2. Why does ice feel cold if it absorbs heat?
It takes heat from your hand, making your hand feel cold.
3. Is freezing water endothermic?
No. Freezing is exothermic because heat is released to the surroundings.
4. What is a simple trick to remember endothermic vs exothermic?
Endo = Enter heat; Exo = Exit heat.
5. What phase changes are endothermic?
Melting, boiling, evaporation, and sublimation are endothermic.
Conclusion
Now you can confidently answer: is ice melting endothermic or exothermic?
Ice melting is endothermic because it absorbs heat to turn from a solid into a liquid. Understanding the difference between endothermic and exothermic helps you in school science, real-life observations, and even exams.
Whenever heat goes in, think endothermic. Whenever heat comes out, think exothermic.
With these simple tricks, memory hacks, comparison tables, and real examples, you can explain these terms to anyone—even a child.
Keep practicing by watching what heat does in daily life. Your science skills will grow every day!

Mira Loxley is a passionate language and writing expert at Definevs.com, turning tricky words and grammar into clear, engaging guides for every reader.









