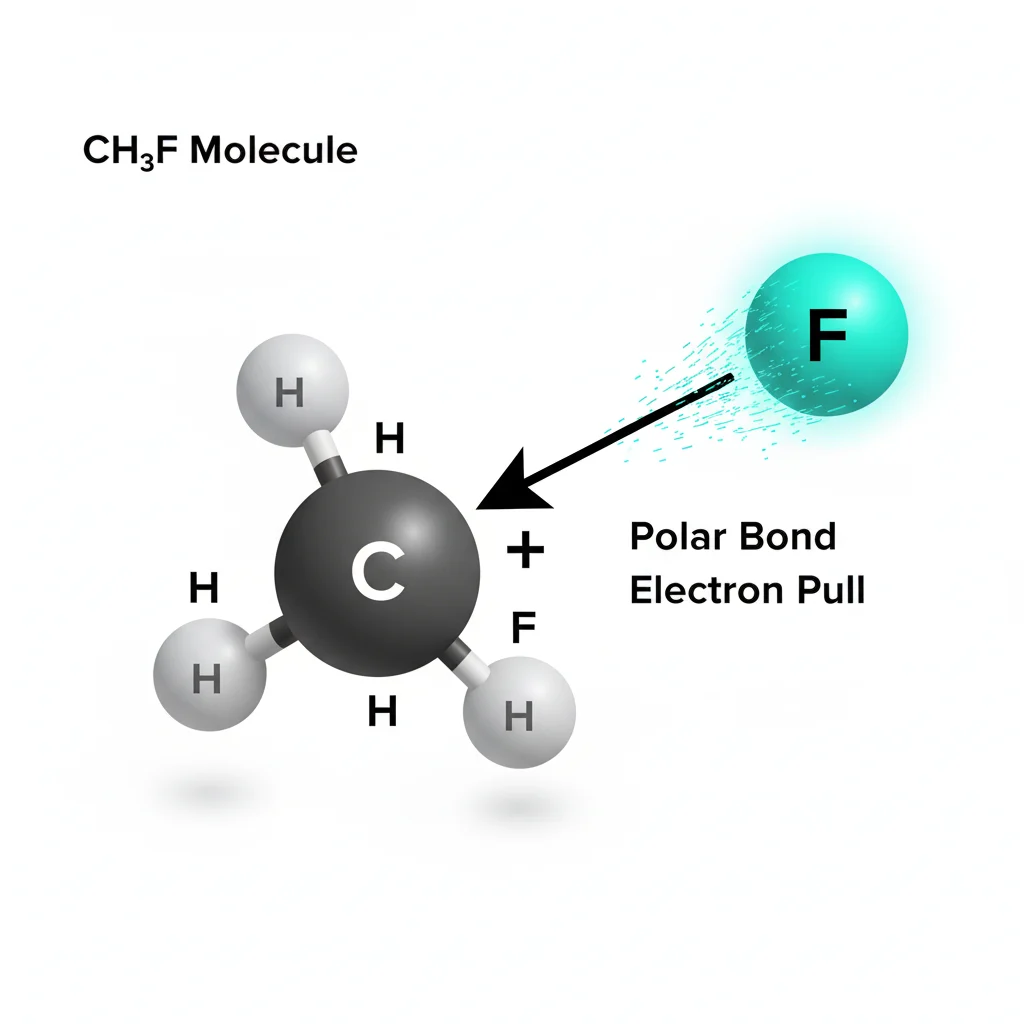
CH₃F is polar, not nonpolar.
It is polar because fluorine pulls electrons strongly, creating an uneven charge distribution in the molecule.
Have you ever stared at a chemistry question like “Is CH₃F polar or nonpolar?” and felt totally confused? You’re not alone! Many students mix up polarity because molecules look simple but behave differently.
This guide will make everything super easy — even if you’re in Grade 4 or completely new to chemistry. We’ll explain what polar and nonpolar mean, how CH₃F behaves, why it behaves that way, and how to tell the difference quickly.
By the end, you’ll know:
- What polar and nonpolar mean
- Whether CH₃F is polar or nonpolar
- How to understand its structure
- Simple examples you’ll never forget
- A comparison table
- Common mistakes students make
This guide is beginner-friendly, clear, and packed with examples anyone can understand!
What Does “Polar” Mean? What Does “Nonpolar” Mean?
What Does Polar Mean?
A polar molecule has uneven sharing of electrons. This creates a “positive side” and a “negative side,” like a tiny magnet.
Easy Examples of Polar Molecules
- Water (H₂O)
- Hydrogen fluoride (HF)
- Ammonia (NH₃)
Think of a polar molecule like a seesaw with one big kid and one small kid — the weight is uneven.
What Does Nonpolar Mean?
A nonpolar molecule shares electrons evenly. There are no positive or negative sides.
Easy Examples of Nonpolar Molecules
- CO₂
- CH₄ (methane)
- N₂
A nonpolar molecule is like a seesaw with two kids of the same size — balanced and equal.
The Key Difference Between Polar vs Nonpolar (Applied to CH₃F)
| Feature | Polar Molecule | Nonpolar Molecule |
|---|---|---|
| Charge Distribution | Uneven | Even |
| Polarity | One + end, one – end | No poles |
| Example | CH₃F, H₂O | CH₄, CO₂ |
| Shape Effect | Asymmetrical | Symmetrical |
| Electronegativity Difference | Big | Small or zero |
Quick Tip to Remember
If a molecule has fluorine (F), oxygen (O), or nitrogen (N) attached — it is very likely polar.
Common Mistakes and How to Avoid Them
❌ Mistake 1: Thinking CH₃F is nonpolar because CH₄ is nonpolar
Why it’s wrong:
F (fluorine) is much more electronegative and pulls electrons strongly.
❌ Mistake 2: Ignoring molecular shape
CH₃F is not symmetrical. Fluorine sits on one side, making it polar.
❌ Mistake 3: Only looking at numbers
Polarity depends on electronegativity + shape, not just values.
When to Call a Molecule Polar
Use polar when the molecule has:
- A strong electronegativity difference
- An uneven shape
- A “pull” toward one atom
Example Sentences
- “Water is polar because oxygen pulls electrons strongly.”
- “HF is polar because the bond is uneven.”
- “CH₃F is polar because fluorine creates a negative side.”
- “Polar molecules mix well with other polar molecules.”
When to Call a Molecule Nonpolar
Use nonpolar when:
- Electrons are shared evenly
- The shape is symmetrical
- No side carries a stronger pull
Example Sentences
- “Methane is nonpolar because all sides match.”
- “CO₂ is nonpolar because it is a straight line.”
- “Nonpolar molecules dissolve in oil but not water.”
- “If atoms pull with equal strength, the molecule is nonpolar.”
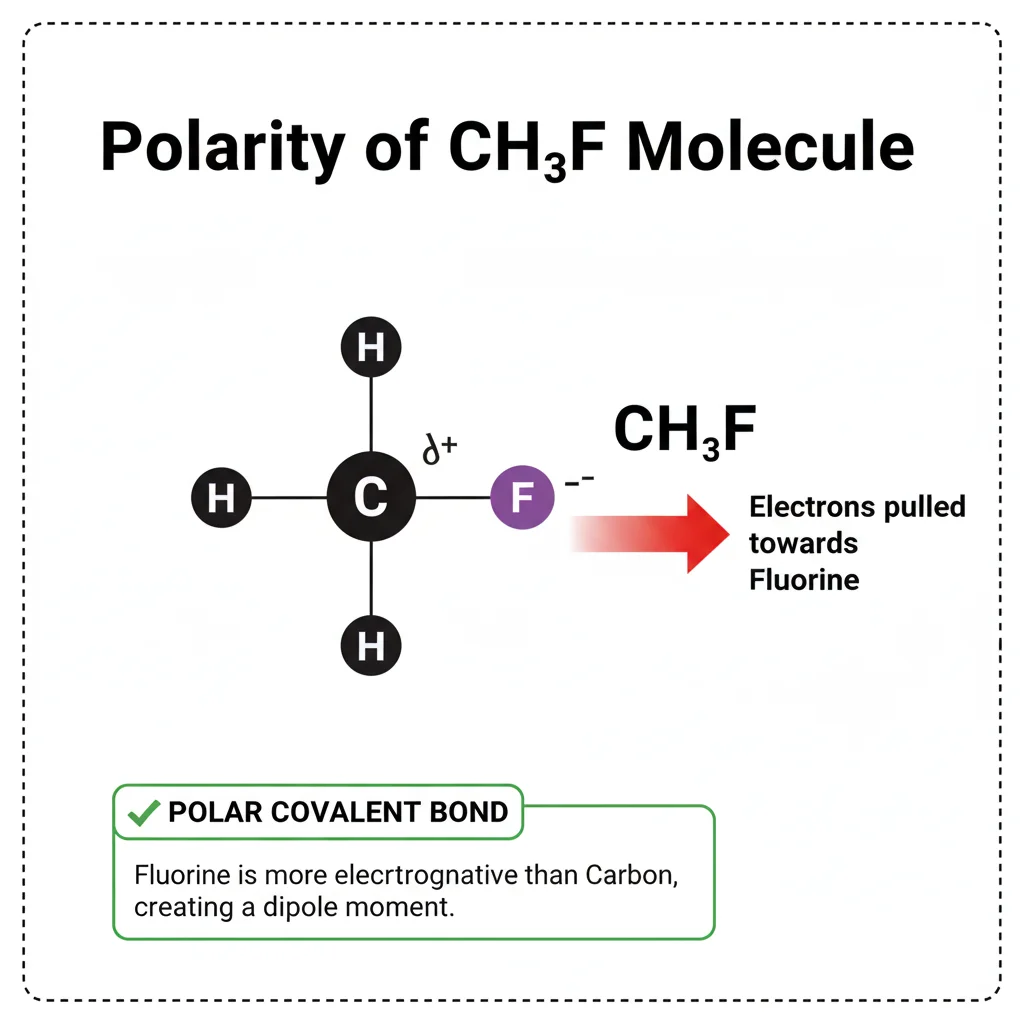
Quick Recap: Is CH₃F Polar or Nonpolar?
- CH₃F is polar.
- Fluorine is highly electronegative.
- The molecule is not symmetrical.
- One side becomes negative (F), the other becomes positive (CH₃).
- This creates a dipole.
Easy memory hack:
👉 “F stands for Fierce Puller — any molecule with fluorine is almost always polar.”
FAQs
1. Is CH₃F polar or nonpolar?
CH₃F is polar because fluorine pulls electrons strongly, creating an uneven charge distribution.
2. Why is CH₃F polar?
Because fluorine is much more electronegative than carbon or hydrogen, so it pulls electrons to its side.
3. Is CH₄ polar or nonpolar compared to CH₃F?
CH₄ is nonpolar, but CH₃F is polar. Adding fluorine breaks the symmetry.
4. Does fluorine always make a molecule polar?
Almost always — fluorine is the strongest electron-pulling atom.
5. Is CH₃F symmetrical?
No. Fluorine sits on one side, making the molecule uneven and polar.
✏️ Mini Quiz
Fill in the blanks:
- CH₃F is ______.
- A polar molecule has an ______ charge distribution.
- CH₄ is ______ because it is symmetrical.
- Fluorine pulls electrons ______.
- If a molecule has fluorine, it is most likely ______.
(Answers: polar, uneven, nonpolar, strongly, polar)
Conclusion
Now you clearly understand whether CH₃F is polar or nonpolar — and why! We explored what polarity means, how to compare polar vs nonpolar, and how fluoride changes the behavior of a molecule. With simple examples, tables, and quick tips, you can now identify polarity in many molecules easily.
Keep practicing, stay curious, and your chemistry skills will grow stronger every day!I

Mira Loxley is a passionate language and writing expert at Definevs.com, turning tricky words and grammar into clear, engaging guides for every reader.